Fort Worth-based AyuVis receives FDA Fast Track approval for its drug for babies with chronic lung disease » Dallas Innovates
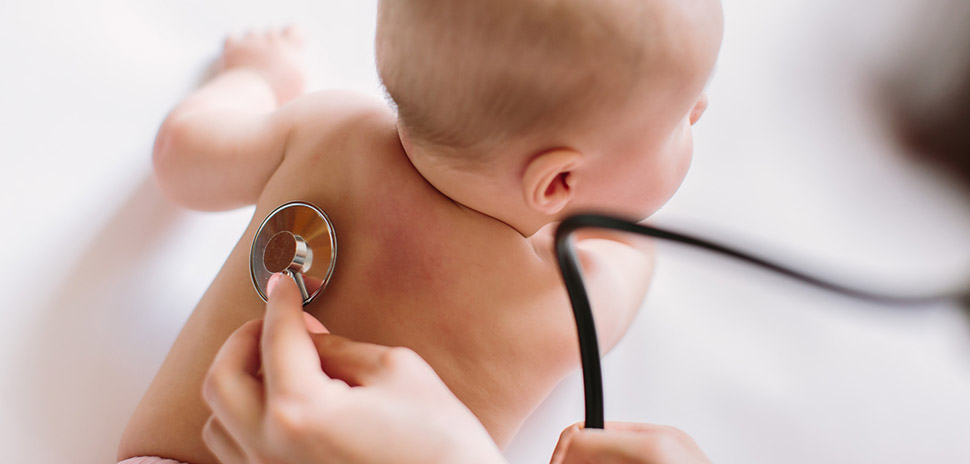
Fort Worth-based AyuVis Research, a clinical-stage biopharmaceutical company, has received Fast Track designation from the U.S. Food and Drug Administration for its new investigational drug AVR-48 for the prevention of bronchopulmonary dysplasia (BPD) – a rare chronic lung disease in children.
AyuVis, a member of TechFW, was founded in 2014 by CEO Suchi Acharya, PhD, and co-founder Gagan Acharya.
The accelerated approval of its drug is a big step for AyuVis. FDA approval is intended to accelerate clinical development and regulatory review of drugs that have the potential to treat serious diseases and address unmet medical needs – with one main goal: to bring groundbreaking new medicines to patients sooner.

(Images: AyuVis)
The approval gives AyuVis access to regular meetings and written communications with the FDA during the drug’s clinical development. In addition, AVR-48 may be eligible for accelerated approval and/or priority review, the company said.
“We are very excited about the progress of our project in clinical trials,” CEO Acharya said in a statement. “Receiving all three awards – Orphan Drug, Rare Pediatric Disease and Fast Track Designation – consecutively is a great testament to our team’s diligent efforts to advance the development of AVR-48 and bring preventive therapy to the newborns who need it most.”
An urgent need: There are currently no FDA-approved therapies for BPD
There are currently no FDA-approved therapies available to prevent or treat BPD, AyuVis noted. Premature infants diagnosed with moderate to severe BPD at 36 weeks (corrected gestational age) or at discharge from a neonatal intensive care unit “often have permanent lung damage from inflammation or oxygen toxicity,” AyuVis said.
Once discharged, the baby is at risk of re-hospitalization, delayed brain development and respiratory problems throughout childhood. BPD can lead to death or lifelong complications such as asthma and chronic obstructive pulmonary disease (COPD), AyuVis added.
Dr. David Riley, chief medical officer of AyuVis and a practicing neonatologist, said neonatologists have been struggling with borderline personality disorder for decades as the survival rates of “extremely premature infants” have improved.
“With AVR-48, we finally have a potential therapy that can treat the root cause of the disease,” Riley said in a statement. “I am excited to see how the drug progresses through the clinical and regulatory process and, if approved, will be eager to bring it to the bedside to improve outcomes for the most vulnerable babies.”
AyuVis said the company is also developing a new generation of immunotherapies that provide “a balanced immune response” with “mild activation and controlled suppression of the immune system” needed to effectively treat BPD.
Earlier this year, the FDA approved the Investigational New Drug Application for AyuVis to proceed with a double-blind, placebo-controlled Phase 1 single ascending dose (SAD) and multiple ascending dose (MAD) study to evaluate the safety and tolerability of AVR-48 in healthy adult volunteers, the company said. The study is expected to begin in 2024 and will be funded in part by grants from the NIH. AyuVis said the Phase 1 clinical trial is the first step in evaluating the safety of its novel and proprietary Macrohage Modulating Platform (MMP) technology in humans, followed by clinical studies on premature babies with borderline personality disorder.

Dr. Suchismita Acharya, co-founder and CEO of AyuVis (Photo: AyuVis)
Received a $2.1 million NIH grant in May
In May, AuyVis received a $2.1 million research grant from the National Heart, Lung and Blood Institute to fund a first-in-human Phase 1 clinical trial for a new generation of immunotherapies, including AVR-48.
It was the fourth NIH grant that AyuVis had received since its founding in 2014, doubling the total funding to $4.2 million. The NHLBI is the third largest NIH institute in the U.S. Department of Health and Human Services.
In 2022, AyuVis received a $1.8 million Small Business Innovation Research Grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to combat a chronic lung disease that is the second leading cause of death in premature babies.
In early 2021, the company received orphan drug designation for AVR-48 from the FDA for the first time.
![]()
Add yourself to the list.
Dallas innovates every day.
Sign up to stay updated daily on news and events in Dallas-Fort Worth.