Surprising mechanism for removing dead cells identified
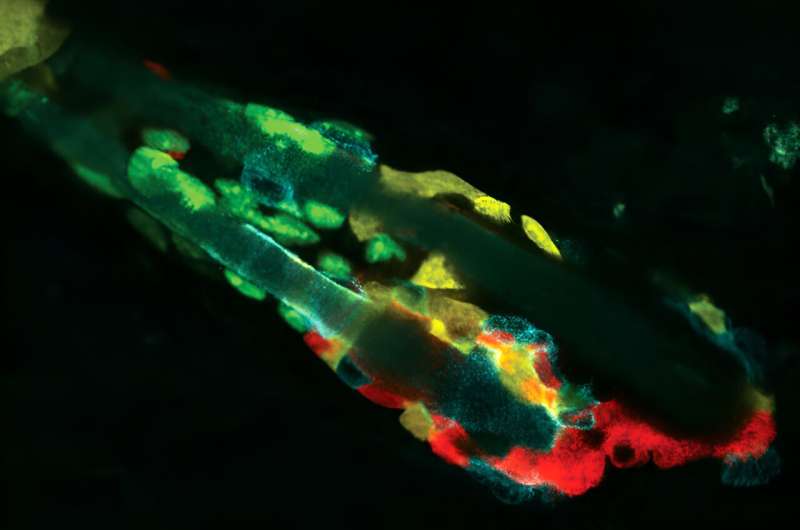
When researchers knocked out the RXRa receptor in a hair follicle, stem cells (green) could no longer ingest dying cells and debris (orange), leading to a buildup of waste products that even professional phagocytes (purple) could not completely remove. Image credit: Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development at Rockefeller University
Every day, billions of our cells die to make room for new cells to grow. Most of these dead cells are eliminated by phagocytes – mobile immune cells that migrate to where they are needed to engulf problematic substances. However, some dying or dead cells are consumed by their own neighbors, natural tissue cells with other primary roles. How these cells sense the dying or dead cells around them is largely unknown.
Now, researchers at Rockefeller University have shown how the sensor system works in hair follicles, which have a familiar cycle of birth, decay and regeneration initiated by hair follicle stem cells (HFSCs). In a new study published in NatureThey show that two sensors work together to receive signals from both dying and living HFSCs, remove debris before tissue damage can occur, and stop functioning before healthy cells are consumed.
“The system appears to be spatially tuned to the presence of corpses and only works if each receptor receives the signal it is tuned to,” says lead author Katherine Stewart, a research associate in the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development at Rockefeller. “If one of them disappears, the mechanism stops working. It’s a really nice way to keep the area clean without using up healthy cells.”
“By focusing their attention on feeding on their dying neighbors, HFSCs keep pro-inflammatory immune cells away,” says Elaine Fuchs, head of the lab. “They probably benefit from those extra calories, too, but once the debris is cleared away, they must quickly return to their job of maintaining the stem cell pool and producing body hair.”
Follow the cycle
Every single hair follicle on your head goes through a specific cycle: growth, destruction, resting and – as seen in your shower drain – hair loss.
To initiate the process, hair follicle stem cells (HFSCs) located in the “bulge” of the upper root sheath of the follicle send signals to epithelial and mesenchymal cells, stimulating growth. This phase takes time and lasts two to six years.
The destructive or catagen phase that follows is short but intense, destroying about 80% of the hair follicle in just a few weeks. The process starts at the follicle base and works its way up toward the HFSC niche. The result is a mass of dying and dead cells that must be removed to prevent the resulting decay from triggering inflammatory or autoimmune reactions.
Normally this would be the job of phagocytes such as macrophages, but there are few of these in the hair follicle, meaning they must be left to local epithelial cells to keep order. Stewart wanted to determine the chemical communication that controls this process.
Dynamic duo
She and her colleagues took a closer look at the catagen phase in hair follicles of mice, whose hair cycle is short and synchronized throughout the hair coat. It is only in the later stages of the catagen phase that the death signals emanating from the base of the follicle finally reach the site where undifferentiated stem cells are located. For a long time it was believed that stem cells were spared from destruction, but surprisingly the team found that some actually die – and are devoured by their neighbors.
Stewart discovered that emptying can only begin when two receptors are activated in the healthy cells. The first, called RXRα, detects the presence of lipids, one of several known “find me” signals secreted by a dying cell. The second, called RARγ, detects growth-promoting retinoic acid secreted by healthy cells.
Neither can activate the cleaning process on its own. “A dying cell triggers the mechanism, and when there are no dead cells left, the lipid signal disappears and all that’s left is the retinoic acid signal from the healthy cells,” Stewart says. “That tells the program to turn itself back on. It’s so elegant in its simplicity.”
They also documented that macrophages were slow to migrate to the region, not appearing until four days after cell death. “It was generally believed that professional phagocytes would eventually invade and do the heavy cleanup work, and that immobile cells were a sort of backup system,” she says.
“I was very surprised to find that the hair follicle stem cells were actually the first to respond, especially because mouse skin is pretty well equipped with macrophages, so they’re not that far away.”
They also found that tissue damage occurred when HFSCs were prevented from clearing dying cells and left the work to macrophages, raising the possibility that genetic defects in this process could contribute to human skin conditions such as inflammation and hair loss.
Positive or negative?
The HFSCs that consumed dying cells nearby – some ate up to six of their neighbors – could benefit from ingesting the cells’ proteins, nucleic acids, solutes and lipids. If so, the benefits remain to be explored – a topic the Fuchs lab will investigate in the future.
“It’s possible that they’re using this material to fuel their own growth or benefit from it in some other way,” Stewart says, “but it’s also possible that it’s having negative effects. Maybe they’re too busy processing all this material to take care of their normal duties.”
The findings are also relevant beyond the hair follicle, as this is just one of several areas of the body where there are few professional phagocytes. In regions of the brain, breast and lung, for example, epithelial and mesenchymal tissue cells, including stem cells, act as replacement phagocytes.
“We often use the phrase ‘you are what you eat,'” adds Fuchs. “For our body’s stem cells, this could be their way of keeping tissue fit by naturally sorting out dying cells and preventing inflammation.”
Further information:
Elaine Fuchs, stem cells strictly regulate the elimination of dead cells to maintain tissue health, Nature (2024). DOI: 10.1038/s41586-024-07855-6. www.nature.com/articles/s41586-024-07855-6
Provided by Rockefeller University
Quote: Surprising mechanism for removing dead cells identified (21 August 2024), accessed 21 August 2024 from https://phys.org/news/2024-08-mechanism-dead-cells.html
This document is subject to copyright. Except for the purposes of private study or research, no part of it may be reproduced without written permission. The contents are for information purposes only.

